LabSolutions™ AG
Соответствие стандартам по испытаниям механических характеристик фармацевтической продукции и медицинского оборудования
- Описание
- Features
Описание
Providing Confident, Secure Data Management on a Network System!
Supports Compliance with Regulations and Guidelines for Mechanical Characteristics Tests in the Pharmaceutical and Medical Equipment Industries
Secure, Confident Data Management
- Mistakes are avoided thanks to database management
- Robust security
Comfortable Operating Environment
- Integrated management of a variety of analysis instruments
- Quickly search through voluminous amounts of data
Heightened Management Productivity
- Consolidated server-based management of users and other system information
- Management of related information for each project
Total Support for Regulatory Compliance
- Functionality supports CSV (IQ/OQ validation) activities
- Supports document creation
The Autograph precision universal testing machines are now compatible with the latest in data integrity.
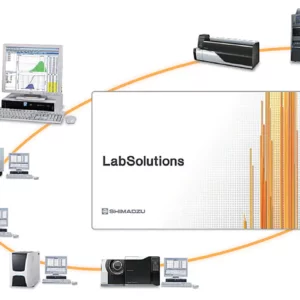
Connecting TRAPEZIUM X-V to the LabSolutions system, which provides ER/ES regulatory compliance, enables confident, reliable data management.
In addition to Autograph data, consolidated management is available for LC, GC, and UV data.
Data integrity refers to the assurance that all the data has been collected, and that it is free from defects or non-conformities. In other words, it is necessary to present not only the data itself but also the meta data (conditions settings, data analysis and other results of processes in which human hands have intervened) in a form that can be clearly seen, for verication together with the data. This is provided by Report Set.
Features
Supports Improved Data Reliability in Materials Industries
Both LabSolutions CS, with its network system structure, and LabSolutions DB, with its stand-alone structure, are available.
Features
Supports Improved Data Reliability in Materials Industries
Both LabSolutions CS, with its network system structure, and LabSolutions DB, with its stand-alone structure, are available.
LabSolutions DB supports improved data reliability through database mediated data management, user management, and management of change logs of a stand-alone system on a single PC.
LabSolutions CS enables server-based integrated management of Autograph test data files together with data files from analysis instruments connected to the system. Test results and sampling data are saved together with operational logs and conditions change logs. Managers can check the logs and add their electronic signatures to the saved data.
The popular TRAPEZIUMX-V is used as the Autograph control software, so most operations are all familiar to the operator, which enables highly reliable materials testing data.
Compatible Autograph Models and Software
Compatible with the AGX-V, AG-X, AGS-X, and EZ-X
Compatible with single software and control software
Note: Not compatible with cycle software.
Note: Some of the functionality is limited in comparison with the regular TRAPEZIUMX-V. For details, refer to the specifications.
Software Comparison
| Name | Standard TRAPEZIUMX-V Software |
LabSolutions DB AG Stand-Alone System |
LabSolutions CS AG Network System |
|---|---|---|---|
| Data Management Method | The data is saved in a local folder on the PC and managed. | The data is saved in the LabSolutions database and managed. | |
| Data Reference | The files in the local folder on the PC | The files in the database | |
| LabSolutions Database | Not available | Available (The database is on a local PC.) | Available (The database is on the server.) |
| User Management | The functions in TRAPEZIUMX-V can be used. | The functions in LabSolutions can be used. | |
| Management of Authority Groups | The functions in TRAPEZIUMX-V can be used. | The functions in LabSolutions can be used. | |
| Project Management | Not available | Capable of project management | |
| Stand-alone/Network | Available as stand-alone only | Available as stand-alone only | Available as network only |
| Data Backup | This is performed using Windows® Explorer on a file by file basis. | This is performed for each project. | |
Note: The standard TRAPEZIUMX-V software is the le management edition. It is not compatible with FDA 21 CFR Part II.
LabSolutions and AGX are trademarks of Shimadzu Corporation.
Windows is either a registered trademark or a trademark of Microsoft Corporation in the United States and/or other countries.