ASAPrep LC/MS Purification Solutions
Описание
LC/MS Purification Solutions
A unique solution to automate and optimize compound purification and isolation
Compound isolation, purity analysis, and impurity analysis in pharmaceutical drug development presents increasing challenges to the industry as there is a clear need to enhance productivity and ensure compliance with evolving regulatory requirements. Such challenges are common to the development of new chemical entities, generic pharmaceutical manufacturing and contract research organizations. To help enhance productivity in compound purification and isolation Shimadzu has developed ASAPrep (Automated Scale-up from Analytical to Preparative), a unique solution to automate and optimize the purification process.
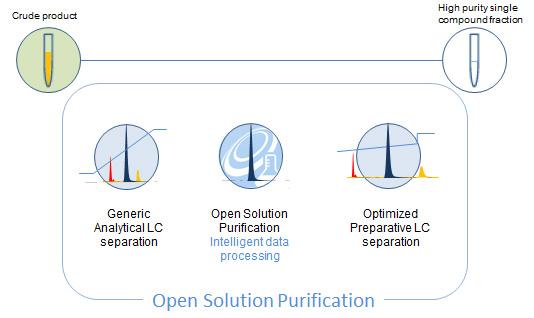
Open Solution Purification
Shimadzu Open Solution Purification software has been co-developed with pharmaceutical scientists to enable an automated approach to walk-up sample submission and optimized analytical to preparative scale up for compound purification and isolation. Using embedded data processing parameters, the software automatically checks the spectral integrity of the target compound for possible co-elution and also looks for closely eluting peaks. If the target compound is sufficiently resolved from other compounds in the sample it is submitted for a focused gradient preparative LC purification to optimize purity and recovery. Samples with co-elution or partial separation close to the retention space of the target compound are highlighted for data review and are not automatically submitted for purification.
Accelerator technology for compound purification
Open Solution Purification software helps to streamline the analytical to preparative scale up process by making smart decisions about the data and the likely outcome. It is designed to deliver the highest purity and recovery of a collected preparative fraction.
Streamlining your workflow
Shimadzu’s new purification software automates the process of purification and achieves the highest productivity. Embedded data processing parameters transform generic analytical LC separation data into fully optimized methods for preparative LC isolation meeting the demands of your laboratory. The system is pre-packaged with the L-Column ODS 2 technology and requires no method development allowing users to quickly generate high purity compound isolation. (Other column chemistries can also be supported).
ASAPrep workflows
Open Access software for compound purification
Step 1
Generic analytical LC screening
Using an open access workflow, analysts simply select a preconfigured analytical LC/PDA/MS method, submit their sample for analysis and review the results using Open Solution Purification software.
System managers can design the system to support multiple generic analytical LC methods including pH switching. The pH switching support includes options for column parking and extensive detox washing to help column management and extend column life time.
Step 2
Reviewing generic analytical LC and submitting samples for preparative LC isolation
Shimadzu’s purification software automatically processes the generic analytical LC data and checks for co-elution or partial separation.
A color coded results table helps to accelerate decisions;
- Green
No co-elution has been detected and the analysis can go forward for preparative isolatio - Amber
Data review — the target compound retention space is compromised by another compound(s) - Red
No co-elution has been detected and the analysis can go forward for preparative isolation
Generic analytical LC method screening
The software has also been designed to support pH switching methods.
Dependent upon the chemical space being considered, it may be necessary to review the effects of pH on the target compound isolation.
Analysts can review the analytical LC data and select the best separation for preparative LC isolation.
All column washes and pre-conditioning methods are built into the process to extend column life times and ensure stability in the LC separation.
(In this example, the high pH separation resulted in less peak tailing compared to low pH method
Generic analytical LC data review
The analytical LC results review allows analysts to see all chromatographic data including mass spectrum and PDA scan data.
If the target compound has a spectral integrity score above a user defined threshold it is considered to be a single compound (a value close to 1 indicates a single compound is present over the retention window of the target compound) . In the case of a partial LC separation, the spectral integrity score will be lower as the mass spectrum scan data across the target compound will not be consistent.
Reviewing preparative LC isolation
The fraction results browser allows analysts to simply track samples and fractions reviewing the LC/PDA/MS data for each collected fraction. Each data stream is shown for prep LC/PDA/MS analysis together with a color coded autosampler plate and fraction collector.
Color coded autosampler tray;
- Green
Collected target compound purity is above a user defined threshold - Amber
Collected target compound purity does not meet all user defined thresholds and requires review - Red
The fraction collected fails to meet the pre-defined criteria
Report generation
A single click report can be generated from the fraction results browser showing the collected fractions and corresponding PDA/MS data.
A pdf report can also be generated as a single click operation.
ASAPrep Optimized purification
A unique algorithm for compound isolation and purification
ASAPrep initially verifies the retention time of the target compound using the analytical-scale LC data. The retention time of the target compound is then used by the ASAPrep algorithm to calculate the focused gradient profile for the preparative-scale separation to deliver the highest purity and recovery of the collected LC fractions.
In the example shown below, the ASAPrep algorithm has been used to change the retention time of each target compound in the preparative scale separation to elute at ~4.3 minutes.
ASAPrep Maximize purity and compound recovery on a single platform
Maximize purity and compound recovery on a single platform
Brochure download
Download the latest brochure.